The periodic table is often regarded as the cornerstone of chemistry. It is a systematic arrangement of the chemical elements, organized based on their atomic number, electron configuration, and recurring chemical properties. This table provides a useful framework for analyzing chemical behavior and is a critical tool for both chemists and students alike. From understanding elemental properties to predicting chemical reactions, the periodic table is indispensable in the study of chemistry.
Historical Development
Early Classifications
Before the periodic table took its modern form, several attempts were made to classify the elements. In 1789, Antoine Lavoisier grouped elements into gases, metals, nonmetals, and earths based on their chemical properties. This was a significant step but still rudimentary by modern standards.
The Law of Triads
In 1829, Johann Wolfgang Döbereiner introduced the Law of Triads, identifying groups of three elements with similar properties where the atomic weight of the middle element was approximately the average of the other two. This hinted at a periodic relationship but was limited to a small number of elements.
The Telluric Helix
In 1862, Alexandre-Émile Béguyer de Chancourtois created the telluric helix, a 3D arrangement of the elements around a cylinder. He noted periodicity in the properties of elements but did not have widespread impact due to the complexity of his model and the inaccessibility of his publication.
Mendeleev’s Periodic Table
The most significant development came in 1869 when Dmitri Mendeleev presented his periodic table. He arranged elements in order of increasing atomic weight and grouped them by similar properties, leaving gaps for undiscovered elements and predicting their properties with remarkable accuracy. Mendeleev’s table gained acceptance because it provided a predictive tool and a coherent framework for understanding chemical relationships.
Modern Periodic Table
The modern periodic table has evolved from Mendeleev’s design, primarily through the work of Henry Moseley in 1913, who rearranged the elements by atomic number rather than atomic weight. This resolved anomalies and aligned with the discovery of isotopes. The current layout includes 118 elements and is periodically updated as new elements are synthesized and discovered.
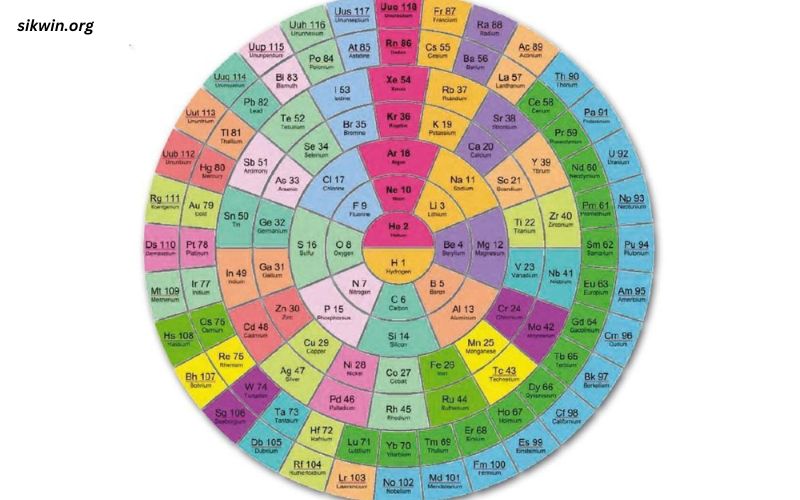
Structure of the Periodic Table
Periods and Groups
The periodic table is divided into periods (horizontal rows) and groups (vertical columns). Each period corresponds to the number of electron shells in the atoms of the elements within that row. As you move across a period, elements gain electrons and protons, increasing their atomic number.
Groups, on the other hand, represent elements with similar chemical properties and valence electron configurations. For example, Group 1 contains the alkali metals, which all have a single valence electron and exhibit similar reactivity.
Blocks of the Periodic Table
The table is further divided into blocks based on the electron configuration of the elements:
- s-block: Groups 1 and 2, including hydrogen and helium. Elements in this block have their outermost electrons in s orbitals.
- p-block: Groups 13 to 18. These elements have their outermost electrons in p orbitals.
- d-block: Transition metals, encompassing Groups 3 to 12, with electrons filling d orbitals.
- f-block: Lanthanides and actinides, where electrons fill f orbitals. These elements are typically placed below the main table to maintain its structure.
Special Groups
- Alkali Metals (Group 1): Highly reactive metals like sodium and potassium.
- Alkaline Earth Metals (Group 2): Slightly less reactive than alkali metals, including magnesium and calcium.
- Transition Metals (Groups 3-12): Metals like iron, copper, and gold with variable oxidation states and a propensity for forming complex ions.
- Halogens (Group 17): Reactive nonmetals such as fluorine and chlorine.
- Noble Gases (Group 18): Inert gases like helium and neon with complete valence electron shells.
Periodic Trends
Atomic Radius
The atomic radius generally decreases across a period from left to right due to increasing nuclear charge pulling electrons closer to the nucleus. It increases down a group as additional electron shells are added.
Ionization Energy
Ionization energy, the energy required to remove an electron from an atom, increases across a period due to stronger nuclear attraction and decreases down a group as electrons are further from the nucleus and more shielded by inner electrons.
Electronegativity
Electronegativity, a measure of an atom’s ability to attract electrons in a chemical bond, follows a similar trend to ionization energy. It increases across a period and decreases down a group.
Electron Affinity
Electron affinity, the energy change when an atom gains an electron, generally becomes more negative across a period, indicating a stronger attraction for electrons, and varies less consistently down a group.
Applications of the Periodic Table
Predicting Chemical Behavior
The periodic table allows chemists to predict the properties and reactivity of elements. For instance, knowing that elements in the same group exhibit similar chemical behavior can help in understanding and predicting the outcomes of chemical reactions.
Designing New Materials
Researchers use the periodic table to design new materials with specific properties by combining elements in novel ways. For example, the creation of superconductors and advanced alloys relies on a deep understanding of elemental properties.
Environmental Chemistry
The periodic table is crucial in environmental chemistry for tracking pollutants and understanding their behavior. Elements like mercury and lead are studied for their toxicological impacts, and their interactions in the environment are predicted based on their position in the periodic table.
Medicine and Pharmacology
In medicine, elements and their compounds are used in diagnostics and treatment. Radioactive isotopes, such as iodine-131, are employed in medical imaging and cancer therapy. The periodic table helps in understanding these elements’ behavior and their safe application in medical practices.
Education
The periodic table is a fundamental educational tool. It is often one of the first concepts introduced in chemistry education, providing students with a structured way to learn about elements and their interactions.
Future Developments
New Element Discovery
The discovery of new elements, particularly those beyond element 118, is an ongoing field of research. These superheavy elements are synthesized in particle accelerators and have very short half-lives. Understanding their properties can provide insights into the limits of the periodic table and the forces that hold atomic nuclei together.
Theoretical Models
Advances in quantum mechanics and computational chemistry continue to refine our understanding of the periodic table. These models help predict the properties of yet-to-be-discovered elements and explain anomalies in the current table.
Sustainable Chemistry
The periodic table plays a role in the development of sustainable chemistry practices. Researchers are exploring elements that can replace more hazardous or scarce materials in industrial processes, aiming to minimize environmental impact and improve sustainability.
Conclusion
The periodic table is more than just a chart of elements; it is a comprehensive guide to the behavior of matter. Its historical development, structural intricacies, and predictive power make it an indispensable tool in chemistry and related fields. As our understanding of the atomic world continues to expand, the periodic table will undoubtedly evolve, continuing to provide a framework for scientific discovery and innovation. Whether in the classroom, the laboratory, or in advanced research, the periodic table remains a symbol of the order and beauty inherent in the natural world.




Only a smiling visitor here to share the love (:, btw great style and design.